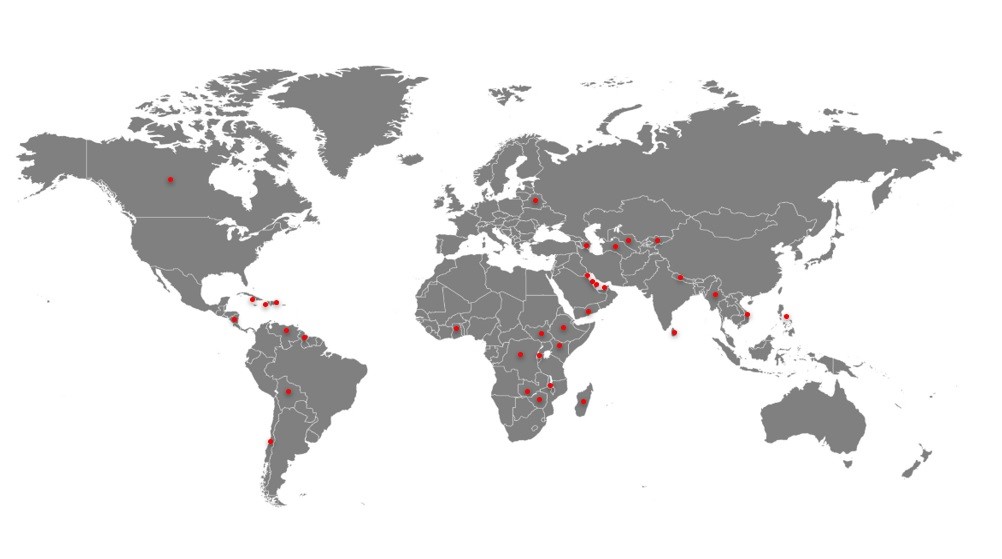
Regulated Markets
CIS
- Bahrain
- Canada
- Kuwait
- Qatar
- UAE
- Belarus
- Turkmenistan
- Uzbekistan
- Kyrgyzstan
- Azerbaijan
Asia
South America
- Myanmar
- Nepal
- Philippines
- Sri Lanka
- Vietnam
- Yemen
- Chile
- Guyana
- Venezuela
- Bolivia
Central America
- Costa Rica
Africa
Caribbean
- Democratic Republic of Congo
- Ethiopia
- Ghana
- Kenya
- Madagascar
- Malawi
- Rwanda
- Uganda
- Zimbabwe
- Burundi
- Haiti
- Jamaica
- Dominican Republic
Meridian always endeavors to manufacture and export innovative and high-quality pharmaceutical products to its clients. Meridian has been in business for more than two decades and is a leader in the suppository segment with a product basket of 30+ molecules and exporting our products to 35+ countries worldwide. We also export external liquids and tablets and have a range of oral liquid and ayurvedic (herbal) products. Our manufacturing facility located in Navsari, Gujarat has been certified by WHO-GMP since 2010 and also has been certified by various international regulatory authorities like Health Canada, UAE, FMHACA Ethiopia, MCAZ Zimbabwe, NDA Uganda, etc. Meridian’s team has the expertise in preparing CTD / ACTD Dossiers as per the need of various markets and have won the trust and confidence of our clients with our consistent quality, and un-interrupted / timely supply, and as a result, Meridian has gained an excellent reputation among its international clients.
In addition to the export of existing products, we have a full-fledged formulation development department that can develop products as per customer specifications and we have already successfully developed various innovative formulations going through the entire development cycle from conceptualization to commercialization.
Meridian looks forward to building long-term associations with partners across the globe for the supply of innovative healthcare products.