
Started operations by renting a factory in Surat to manufacture tablet products for Parke Davis

Purchased vacant plot in GIDC, Navsari and started constructing modern pharmaceutical factory

Small marketing team was formed in Mumbai

Acquired two existing brands: Hallens Glycerin Suppositories and Benzyl Septol antiseptic liquid
Three new and innovative products were developed and launched through a handful of MRs in Mumbai city: Ayurvel, Nasomist, Inhalade

Contract manufacturing of tablets for Cadila Pharmaceuticals
Marketing team expansion from mumbai to rest of Maharashtra & Goa

Established suppository and external liquid departments in Navsari factory

Launched Calcifit Tablets

Launched Flatuna Drops / Tablets

Upgradation of factory including installation of first AHUs

Expanded marketing network to Gujarat and Madhya Pradesh
Launched Tussalyte cough syrup

Started exports

Factory approved by auditors from Pfizer USA

State GMP Certification awarded to factory

Contract Manufacturing for Cipla

Launched Calcifit Syrup
Launched Ferrofit Tablets
Launched Suppol paracetamol suppositories & Nasomist X

Launched Corect haemorrhoidal suppositories

Launched Cofftab lozenges

Upgraded factory to comply to revised 'Schedule M/T' GMP norms including installations of many AHU's
Launched Dipodem Tablet

Expanded marketing network to Chattisgarh
Launched Fensupp diclofenac suppositories

Second floor constructed and major upgradation of factory Awarded by WHO-GMP Certification
New office on lease-setup for export dept.
Launched Tussalyte XP

Expanded marketing network to Andhra Pradesh and Telangana

Implementation of ERP system
Launched Rilistiff roll-on pain reliever

Launched Tussalyte XP Drops, Ferrofit Syrup & Ferrofit Drops
Launched Calcifit D Tablets

Registered products in Hong Kong

Participation in overseas CPHI event
Registered products in Ghana, Malawi & UAE

Factory inspected and approved by PPB, Kenya & FDB, Ghana
Launched Nasomist XP Drops

Upgradation / construction in factory incl. water system
Inspected & approved by DPM, Ivory Coast

Registered products in Myanmar, Turkmenistan & Kenya

Launched Correct-O Tablets
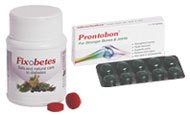
Launched Prontobon and Fixobetes ayurvedic tablets

Installed fully automatic Italian suppository manufacturing machine
Factory inspected and approved by NDA Uganda

Factory inspected and approved by MoH Belarus, MoH Congo & MCAZ Zimbabwe authority

Products registered in Belarus, Congo & Rwanda

Expanded marketing network to Karnataka

Major upgradation project at factory incl. Tablet section as per WHO GMP and entire factory as per PICS.
Installation of ETP Plant.

Factory certified by UL (global auditing company)

Tablet department certified by WHO
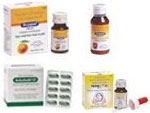
Launched Inhalade-O ayurvedic capsules, Suppol suspension & drops.

Installed 2nd automatic suppository manufacturing machine and various R&D model machineries
Factory inspected and approved by FMHACA Ethiopia, Yemen and Nepal.


Registered products in Kuwait, Vietnam, Ethiopia, Nepal.
Developed unique tablet and external liquid product for Canadian customer

Installed fully automatic external liquid filling machine.

UL Certified and All Production Lines approved by Health Canada.
Products registered in Chile, Costa Rica, Zambi and Zimbabwe.

Launched Ayurvel syrup

